 Elixiron Immunotherapeutics focuses on developing precision-targeted immunotherapy drugs. By leveraging advanced immunological insights, the company designs novel therapies specifically engineered to address disease-causing immune mechanisms. Founded in 2017, Elixiron’s core innovation is rooted in the decades of translational medical research conducted by its three founders in the fields of neuroinflammation, autoimmune diseases, and oncology. The company’s excellence has been recognized with the 2024 Taiwan BIO Award and the 2025 National Innovation Award.
Elixiron Immunotherapeutics focuses on developing precision-targeted immunotherapy drugs. By leveraging advanced immunological insights, the company designs novel therapies specifically engineered to address disease-causing immune mechanisms. Founded in 2017, Elixiron’s core innovation is rooted in the decades of translational medical research conducted by its three founders in the fields of neuroinflammation, autoimmune diseases, and oncology. The company’s excellence has been recognized with the 2024 Taiwan BIO Award and the 2025 National Innovation Award.
Breakthroughs in Neuroinflammation
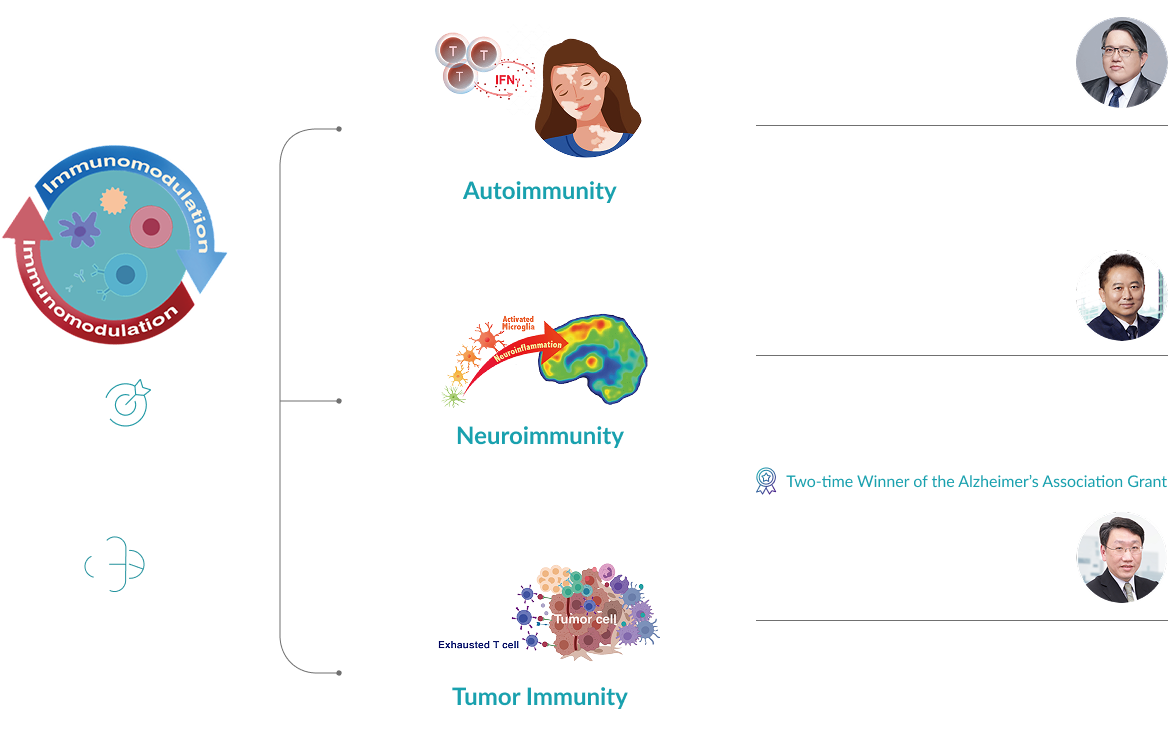
Under the leadership of Chairman and Founder Dr. Hung-Kai (Kevin) Chen, MD, PhD—who brings extensive experience in clinical medicine, academic research, and industrial development—the company is developing Enrupatinib (EI-1071). Currently in Phase II clinical trials for Alzheimer’s disease, Enrupatinib is a highly selective, brain-penetrant, small-molecule CSF-1R inhibitor.
This invention was awarded the Gold Medal at the 2020 National Invention and Creation Awards. Furthermore, it has earned international acclaim and support from the Alzheimer’s Association (USA) in both 2020 and 2022, accelerating its clinical development for neuroinflammation and offering new hope for Alzheimer’s patients worldwide.
Innovations in Autoimmune Diseases
In the field of autoimmune diseases, Co-founder and Chief Scientific Officer Dr. Cheng-Lung Ku leads the development of Indemakitug (EI-001). This novel anti-interferon-gamma ( IFN-γ) antibody was cloned directly from patient cells.
Dr. Ku’s published translational research has identified IFN-γ as a key biomarker and pathogenic factor in vitiligo. Consequently, Indemakitug (EI-001) holds significant potential for treating vitiligo and other IFN-γ-mediated autoimmune conditions. The project has successfully advanced to Phase II clinical trials for vitiligo.
 |
New Clinical IndicationsExpand new indications through clinical translational medicine based on confirmed pharmacological effects.
|
|
Innovative Technology PlatformsBuilding advanced platform technologies to accelerate drug development
|
||
Novel Therapeutic TargetsInternational industry-university collaboration discovers new targets for disease treatment
|
New Clinical Indications |
|
|
In the first phase, through clinical immunology research, Elixiron collects and analyzes extensive clinical data to investigate pathogenic immune mechanisms and identify the optimal combination of drugs and indications. This approach has led to the proposal of novel treatment strategies using precision-targeted immunotherapy for Alzheimer's disease and vitiligo. These efforts have also earned Orphan Drug Designations from the U.S. Food and Drug Administration (FDA) for the treatment of idiopathic pulmonary fibrosis and hemophagocytic lymphohistiocytosis (HLH). |
|
Innovative Technology Platforms |
|
|
In the second phase, Elixiron has developed proprietary platforms, including single B-cell cloning technology, an AI-designed antibody sequence library, T-Action Technology, and a nucleic acid drug development platform. These cutting-edge technologies have significantly accelerated the development of multiple novel drug candidates. |
|
Novel Therapeutic Targets |
|
|
In the third phase, under the leadership of co-founder Professor Mu-Hua Yang, the tumor microenvironment of head and neck cancer has been thoroughly analyzed, revealing key mechanisms of immune suppression. This research has enabled the identification of novel therapeutic targets and the development of innovative targeted immunotherapies. Among them, the dual-functional antibody drug EI-003 was published in the journal Cell Reports Medicine in 2023. |
Elixiron Immunotherapeutics focuses on developing precision-targeted immunotherapy drugs. By leveraging advanced immunological insights, the company designs novel therapies specifically engineered to address disease-causing immune mechanisms. Founded in 2017, Elixiron’s core innovation is rooted in the decades of translational medical research conducted by its three founders in the fields of neuroinflammation, autoimmune diseases, and oncology. The company’s excellence has been recognized with the 2024 Taiwan BIO Award and the 2025 National Innovation Award.
Breakthroughs in Neuroinflammation
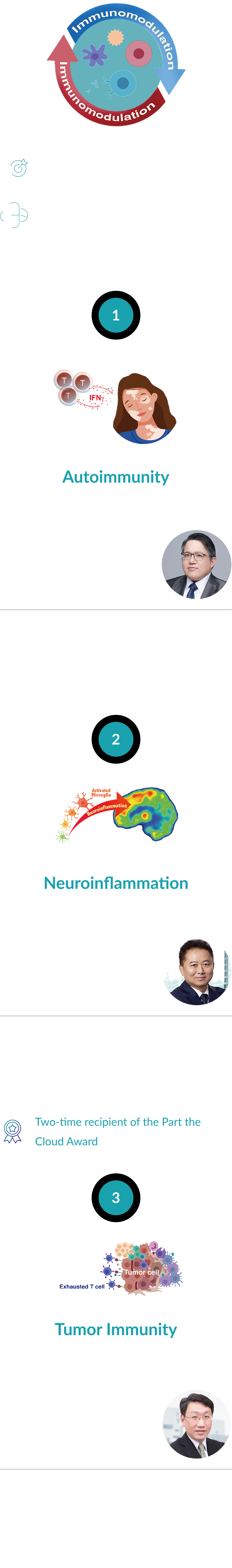
Under the leadership of Chairman and Founder Dr. Hung-Kai (Kevin) Chen, MD, PhD—who brings extensive experience in clinical medicine, academic research, and industrial development—the company is developing Enrupatinib (EI-1071). Currently in Phase II clinical trials for Alzheimer’s disease, Enrupatinib is a highly selective, brain-penetrant, small-molecule CSF-1R inhibitor.
This invention was awarded the Gold Medal at the 2020 National Invention and Creation Awards. Furthermore, it has earned international acclaim and support from the Alzheimer’s Association (USA) in both 2020 and 2022, accelerating its clinical development for neuroinflammation and offering new hope for Alzheimer’s patients worldwide.
Innovations in Autoimmune Diseases
In the field of autoimmune diseases, Co-founder and Chief Scientific Officer Dr. Cheng-Lung Ku leads the development of Indemakitug (EI-001). This novel anti-interferon-gamma ( IFN-γ) antibody was cloned directly from patient cells.
Dr. Ku’s published translational research has identified IFN-γ as a key biomarker and pathogenic factor in vitiligo. Consequently, Indemakitug (EI-001) holds significant potential for treating vitiligo and other IFN-γ-mediated autoimmune conditions. The project has successfully advanced to Phase II clinical trials for vitiligo.
 |
New Clinical Indications |
|
Expand new indications through clinical translational medicine based on confirmed pharmacological effects.
|
 |
Innovative Technology Platforms |
|
Building advanced platform technologies to accelerate drug development
|
 |
Novel Therapeutic Targets |
|
International industry-university collaboration discovers new targets for disease treatment
|
Elixiron Immunotherapeutics’s drug development strategy, known as the “Innovation Trilogy,” comprises three pillars: new clinical indications, innovative technology platforms, and novel therapeutic targets.
1. New Clinical Indications |
|
In the first phase, through clinical immunology research, Elixiron collects and analyzes extensive clinical data to investigate pathogenic immune mechanisms and identify the optimal combination of drugs and indications. This approach has led to the proposal of novel treatment strategies using precision-targeted immunotherapy for Alzheimer's disease and vitiligo. These efforts have also earned Orphan Drug Designations from the U.S. Food and Drug Administration (FDA) for the treatment of idiopathic pulmonary fibrosis (IPF) and hemophagocytic lymphohistiocytosis (HLH). |
2. Innovative Technology Platforms |
|
In the second phase, Elixiron has developed proprietary platforms, including single B-cell cloning technology, an AI-designed antibody sequence library, T-Action Technology, and a mRNA drug development platform. These cutting-edge technologies have significantly accelerated the development of multiple novel drug candidates. |
3. Novel Therapeutic Targets |
|
In the third phase, under the leadership of co-founder Professor Mu-Hua Yang, the tumor microenvironment of head and neck cancer has been thoroughly analyzed, revealing key mechanisms of immune suppression. This research has enabled the identification of novel therapeutic targets and the development of innovative targeted immunotherapies. Among them, the dual-functional antibody drug EI-003 was published in the journal Cell Reports Medicine in 2023. |



