

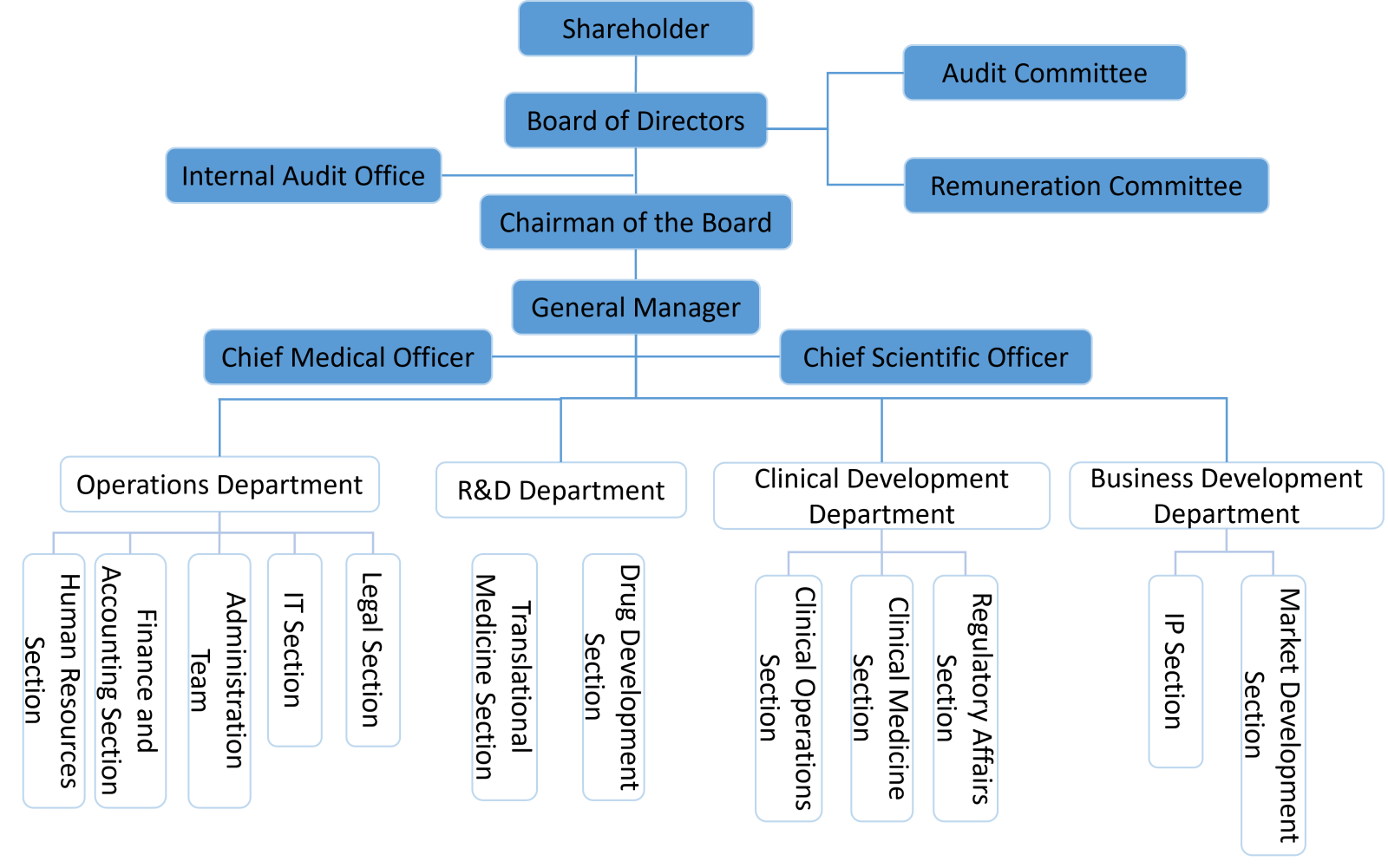
| Department |
Functional Responsibilities |
|
Board of Directors |
Acting as the administrative unit for corporate meetings, we convene regular shareholders' meetings, manage significant matters mandated by the shareholders, and represent shareholders in overseeing the implementation of meeting resolutions. |
|
Chairman of the Board |
Responsible for convening board meetings, managing board-authorized matters, and supervising the management’s implementation of board decisions. |
|
Internal Audit Office |
Conducting audits of internal control designs and implementation across group companies based on the audit plan. This involves identifying deficiencies, evaluating operational efficiency, and providing recommendations for improvement to ensure the continuous effectiveness of internal controls and enhance overall operational performance. |
|
Audit Committee |
|
|
Remuneration Committee |
|
|
Operations Department |
|
|
R&D Department |
|
|
Clinical Development Department |
In accordance with the Regulations on Good Clinical Practice (GCP) and the study protocols, the unit is responsible for the design, regulatory submission, execution, and reporting of clinical trials across all phases. |
|
Business Development Department |
|

| Department |
Functional Responsibilities |
|
Board of Directors |
Acting as the administrative unit for corporate meetings, we convene regular shareholders' meetings, manage significant matters mandated by the shareholders, and represent shareholders in overseeing the implementation of meeting resolutions. |
|
Chairman of the Board |
Responsible for convening board meetings, managing board-authorized matters, and supervising the management’s implementation of board decisions. |
|
Internal Audit Office |
Conducting audits of internal control designs and implementation across group companies based on the audit plan. This involves identifying deficiencies, evaluating operational efficiency, and providing recommendations for improvement to ensure the continuous effectiveness of internal controls and enhance overall operational performance. |
|
Audit Committee |
|
|
Remuneration Committee |
|
|
Operations Department |
|
|
R&D Department |
|
|
Clinical Development Department |
In accordance with the Regulations on Good Clinical Practice (GCP) and the study protocols, the unit is responsible for the design, regulatory submission, execution, and reporting of clinical trials across all phases. |
|
Business Development Department |
|



